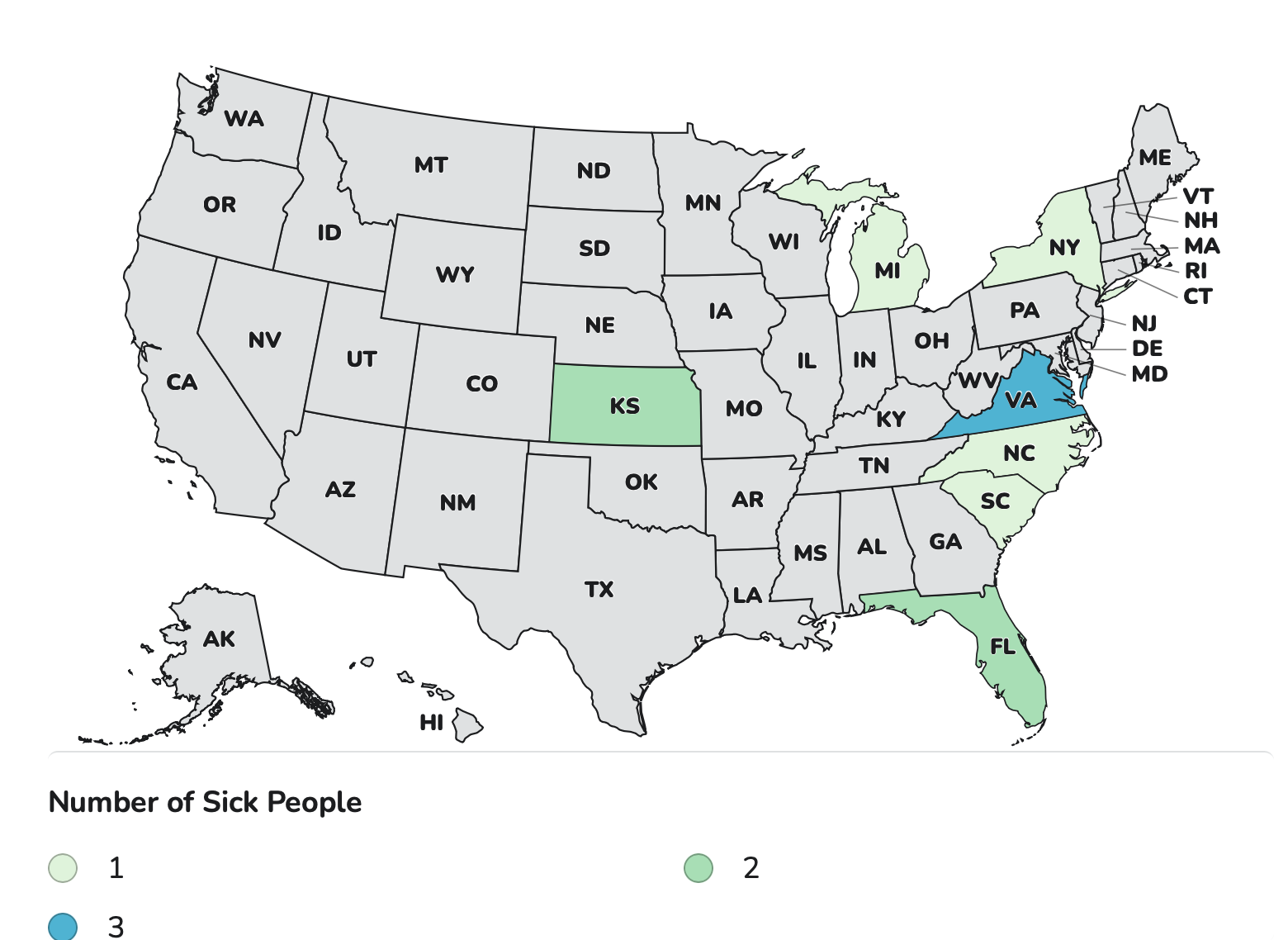
The FDA and CDC have been investigating supplements that have tested positive for poisonous yellow oleander.
As of Nov. 3, the Food and Drug Administration has identified 26 products with the substance, which is sometimes identified as tejocote root, Crataegus mexicana, Raiz de Tejocote, and Mexican Hawthorn. Recalls have been initiated by some of the companies that are selling the products. However, other companies are refusing to issue recalls.
Therefore, the FDA has issued a public warning about the products, which can be found by clicking here.
Toxic yellow oleander and can cause neurologic, gastrointestinal and cardiovascular adverse health effects that may be severe, or even fatal.
- The FDA is advising consumers to stop using and dispose of these products.
- The FDA advises consumers who have taken any of these products to contact their health care provider immediately, even if the products have not been used recently, so that an appropriate evaluation may be conducted.
- Call 9-1-1 or get emergency medical help right away if you or someone in your care has side effects from these products.
In September 2023, the Centers for Disease Control and Prevention published a report of several tejocote root products found to be substituted with yellow oleander, a toxic substance. Based on this report, the FDA initiated an investigation to sample and test additional tejocote root products. As part of FDA’s ongoing surveillance sampling efforts. The FDA is actively working with the third-party platforms where these products are sold.
The FDA’s investigation is ongoing, and the agency will continue to provide information on any further actions as it becomes available. Products may be added to this advisory.
(To sign up for a free subscription to Food Safety News, click here)