The Food and Drug Administration is continuing to investigate several outbreaks, but is reporting little information on most of the investigations.
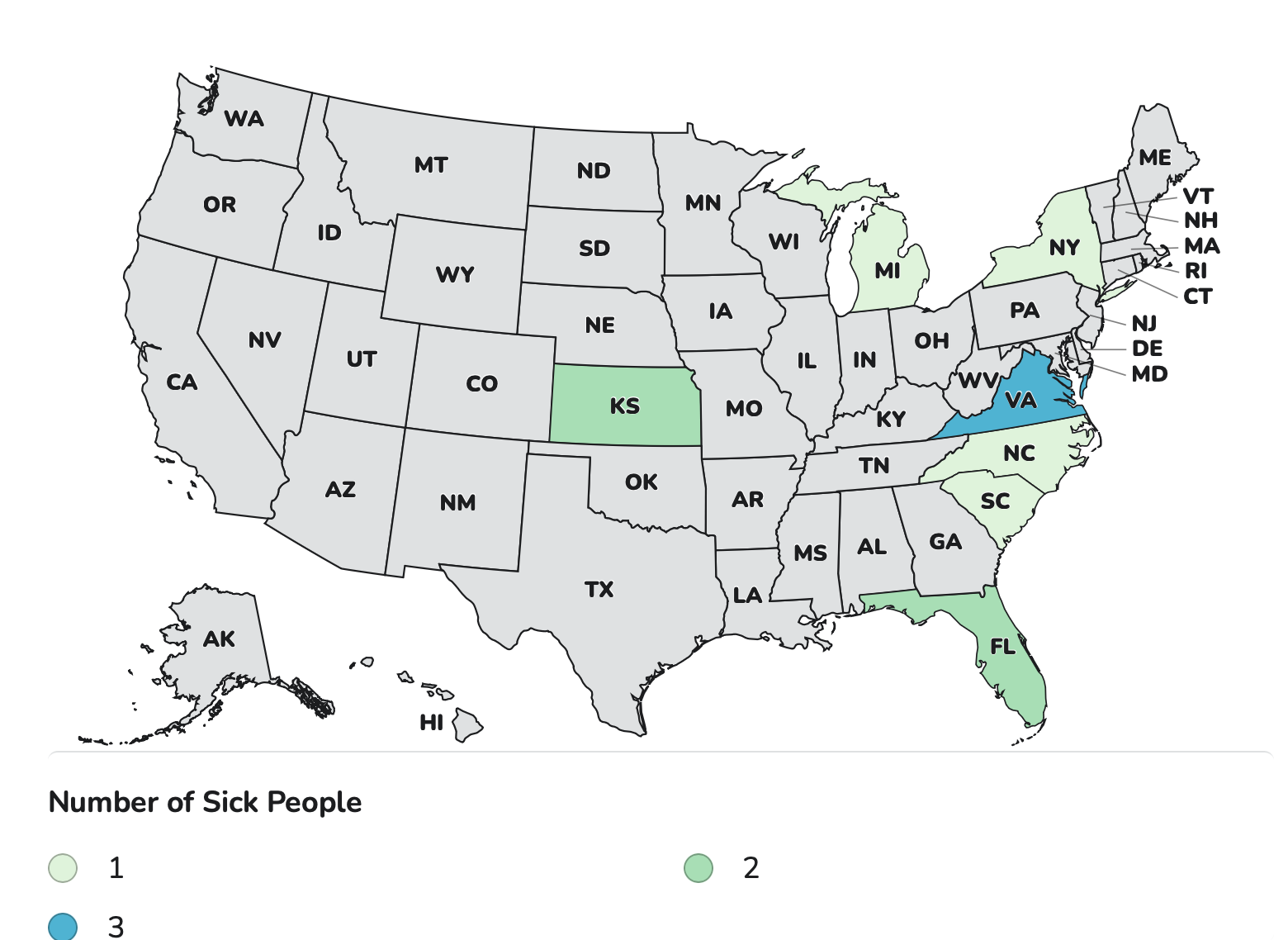
For an outbreak of Listeria infections the FDA has updated its advisory to include additional brands of ready-to-eat pasta meals on the recall list.
As of Sept. 25, a total of 20 people infected with the outbreak strain of Listeriahave been reported from 15 states. Nineteen of the patients have been hospitalized and five of the patients have died. The FDA first reported the outbreak on April 9.
Recalled products related to the outbreak include:
- Giant Eagle smoked mozzarella pasta salad – expiration dates Sept. 30 through Oct. 7 (Giant Eagle recall)
- Kroger stores deli bowtie and penne pasta salads – sold on Aug. 29 through Oct. 2 (Kroger recall)
- Scott & Jon’s Shrimp Scampi with Linguini Bowls 9.6-oz – best if used by dates of 3/12/2027, 3/13/2027, 3/17/2027, 3/21/2027 (Demers Food Group Recall)
- Trader Joe’s Cajun Style Blackened Chicken Breast Fettucine Alfredo 16-oz plastic tray packages with “best if used by” dates of 9/20/2025, 9/24/2025, 9/27/2025, 9/28/2025, 10/01/2025, 10/03/2025, 10/05/2025, 10/08/2025, or 10/10/2025 (USDA FSIS public health alert)
- Albertsons stores recalled store-made deli pasta salads – sell thru dates from Sept. 8 to Oct. 4 (Albertsons recall)
- Marketside Linguine with Beef Meatballs & Marinara Sauce 12-oz. – best if used by dates of SEP 22, 2025; SEP 24, 2025; SEP 25, 2025; SEP 29, 2025; SEP 30, 2025; and OCT 01, 2025 (USDA FSIS public health alert)
- Marketside Grilled Chicken Alfredo with Fettuccine 12.3-oz – best-by date of June 26 or prior; and 32.8-oz – best-by date of June 27, 2025, or prior (FreshRealm recall)
- Home Chef Chicken Fettuccine Alfredo 12.5-oz – best-by date of June 19, 2025, or prior (FreshRealm recall)
On Sept. 25, the supplier of the affected pasta, Nate’s Fine Foods Inc., recalled certain lots of pre-cooked pasta including fettucine, linguine, and farfalle (bowtie), after a sample of linguine pasta collected and tested by FreshRealm tested positive for Listeria monocytogenes.
Nate’s Fine Foods Inc., does not sell affected products direct to retail. The firm is working with the FDA and their customers to determine if additional recalls are needed.
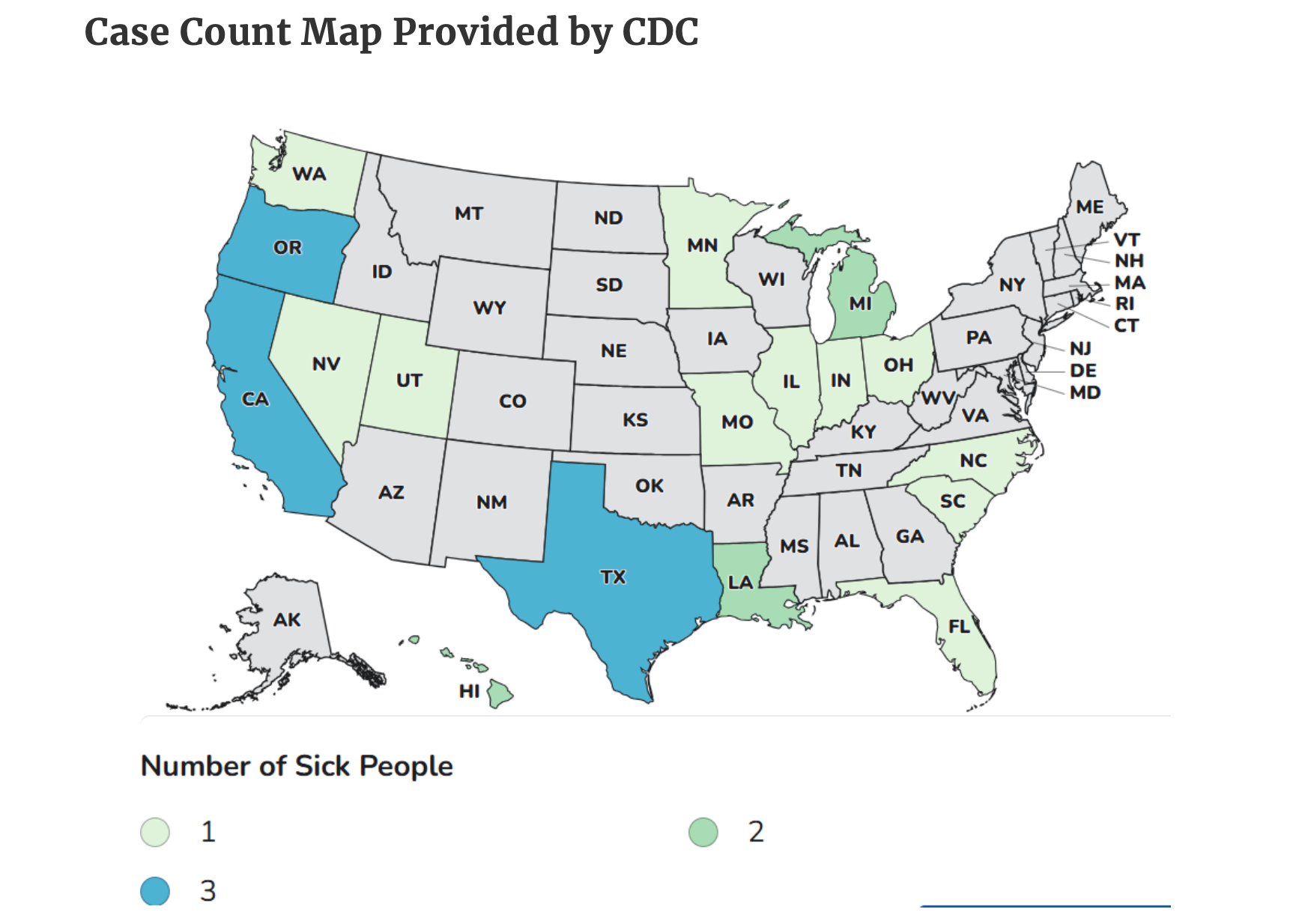
For an outbreak of Salmonella Lomalinda the patient count has increased from 37 to 39 in the past week. The FDA has not reported the patients’ ages or where they live. The source of the pathogen has not yet been determined but the FDA has begun traceback. The agency has not reported what food is being traced. In its outbreak update this week the FDA reported that it has begun an onsite inspection and sample testing, but it is not reporting what location it is inspecting or what it is testing. The FDA first reported the outbreak on Sept. 17.
For an outbreak of Listeria monocytogenes, the patient count remains steady at eight. The FDA has not found a source of the pathogen, but has begun an onsite inspection and sample testing. The agency is not reporting what location it is inspecting or what is being sampled. The agency previously initiated traceback efforts but has not reported what food is being traced. The FDA first reported the outbreak on Sept. 17.
For an outbreak of Listeria monocytogenes infections, the patient count has remained steady at 26. The FDA has not reported the patients’ ages or where they live. A source of the pathogen has not been determined. The agency has begun traceback but has not reported what food is being traced. The FDA first posted the outbreak on Sept. 4.
For an outbreak of Salmonella Enteritidis infections the patient count has remained steady at 45. The FDA has not reported how old the patients are or where they live. A source of the pathogen has not been identified. The FDA has begun trace back efforts but has not reported what food it is tracing. The agency has also begun onsite inspections and sample testing but has not reported what location is being inspected or what it is testing. The FDA first reported the outbreak on Aug. 27.
(To sign up for a free subscription to Food Safety News, click here)